
Case Study: Transforming Clinical Trials: How a Global Pharma Leader Scaled Faster and Strengthened Security with SAM
EXECUTIVE SUMMARY
Building a Modern Foundation for Research
A top 10 global pharmaceutical company, long recognized for its leadership in addressing chronic diseases, is now expanding its mission to tackle emerging health challenges including obesity, rare blood disorders, and endocrine conditions. While their approach centers on pioneering scientific breakthroughs, expanding access to medicines, and working actively to prevent disease, the company found itself constrained, not by its science, but by its technology.

Exostar’s Secure Access Manager (SAM) has allowed the company to overcome its technology challenges and make an impact within the rare disease clinical trial space by streamlining their clinical operations and strengthening their identity security, creating a trusted environment that promotes essential collaboration internally and with research partners.
THE CHALLENGE
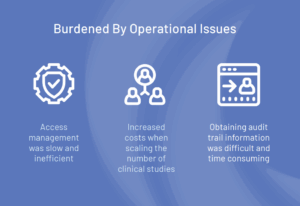
Overcoming Operational Inefficiencies That Hindered Clinical Trial Growth
Despite their scientific innovation, this pharmaceutical company faced significant challenges in engaging research partners with respect to identity security and managing access to clinical trial applications. The organization’s clinical operations were slowed by manual, spreadsheet-based processes for user management and access control.
This inefficient and labor-intensive approach increased the likelihood of non-compliance with industry standard regulations and produced delays in clinical trial applications access that cost valuable time and resources and frustrated partners.

THESE OUTDATED WORKFLOWS ALSO INTRODUCED SIGNIFICANT RISKS:
- Increased burden on clinical operations teams: Manual user management consumed valuable time and diverted focus from high-priority trial activities.
- Greater vulnerability to cybersecurity threats: Password reuse and fragmented identity practices exposed systems to elevated risk of unauthorized access and attacks.
- Difficulty maintaining audit-ready records: Without centralized reporting, compliance efforts were time-consuming and prone to gaps.
- Slower clinical trial execution: Delays in granting access to critical systems hindered study startup and impacted overall research timelines.
In addition, the organization’s partner site users also had to manage multiple passwords for the same systems across various sponsors they collaborated with for their studies. The burden of managing multiple passwords led to password reuse, increasing the risk of vulnerable accounts. Additionally, the use of weak passwords increased the risk of brute-force attacks, making accounts more vulnerable to compromise.
Without modern access management, scaling new clinical trials quickly and securely became increasingly difficult.
THE SOLUTION
Unlocking Seamless, Secure Clinical Access with Exostar’s SAM
To address these challenges, the organization selected Exostar’s Secure Access Manager (SAM): A centralized, vendor-agnostic identity and access management solution built for the life sciences industry, enabling seamless, secure access to clinical applications for sponsors and site users. SAM enhances their identity security posture and streamlines their access management to their clinical trial applications. The organization highlighted Exostar’s vendor agnostic approach as a key differentiator, which allowed the organization to facilitate integrations with Exostar across various clinical trial application vendors.

THE BENEFITS
Delivering Faster Onboarding, Happier Sites, and Stronger Compliance
Implementing Exostar’s SAM delivered immediate and lasting benefits for the pharmaceutical company’s clinical operations:
- Accelerated Operational Efficiency: Automated workflows cut manual tasks and accelerated site user onboarding.
- Scalable Study Growth: Studies scaled faster without needing proportional increases in operational resources.
- Enhanced Site User Satisfaction: Single sign-on eliminated the frustration of multiple passwords, positioning the company as a sponsor of choice among clinical partners.
- Stronger Security and Compliance: Centralized identity management reduced vulnerabilities, while audit reporting became faster and easier.

THE RESULTS
Achieving Measurable Operational Gains
The partnership with Exostar delivered tangible, quantifiable results:

Paving the Way for Future Success
By modernizing its clinical access management with Exostar, this global pharmaceutical leader not only reduced risk and cost—it also laid the foundation for more agile, scalable trials in the future. As they continue to expand into rare disease research, Exostar will remain a trusted partner in ensuring secure, efficient collaboration with clinical sites worldwide – positioning Exostar’s customer as a sponsor of choice and promoting continued success.
