Blog
Key Takeaways on Clinical Trials from DIA

Exostar was excited to attend the DIA 2024 Global Annual Meeting on Charting New Horizons and celebrate the 60th anniversary of the Drug Information Association (DIA). We had many impactful discussions while connecting with industry professionals during this four-day event.
At Exostar, we believe collaboration is essential for developing innovative technologies. This was an excellent opportunity for us to hear from the life sciences community directly on how we can meet your needs with vital, groundbreaking technologies.
After the conference, DIA shared an article from the June issue of the DIA Global Forum on the primary challenges faced by clinical trial sites. Here are our main takeaways on clinical trial site challenges and solutions from their piece, Back to (Communication) Basics: Reducing Site Burden and Establishing a Sponsor/CRO-of-Choice Relationship with Investigative Sites.
DIA Delivers Findings on Urgent Clinical Trial Pain Points
In 2023, Tufts Center for the Study of Drug Development (CSDD) followed up on an earlier survey of clinical trial site pain points with a literature review ranking those challenges by urgency, seen in the first table in the article. After the literature review, Tufts CSDD conducted 22 interviews featuring various site types and staff roles to gather direct feedback on challenges.
Technology Challenges Faced by Clinical Trial Sites
Unsurprisingly, technology improvements ranked at the top of urgent priorities for sites. One staff member interviewed for the survey expressed their exasperation at the number of passwords sites are bogged down with during trials: “Passwords! Don’t even get me started on this. Changing passwords every 30-45 days across multiple systems is a huge burden.” Clunky applications and sign-on processes accumulate into major inefficiencies for sites.
Communication and Trial Initiation Burdens
“Cumbersome feasibility and start-up processes” and “poor communication flow” also appeared as two of the six key themes discovered in interviews with site staff. Start-up struggles include “unrealistic timelines and turn-around expectations,” which are compounded by communication issues, such as “redundant information requests.” A majority of site staff who responded to the 2022 survey also felt that “study start-up, execution and other operating activities have worsened substantially over the last 5 years.”
Opportunities for Improving Clinical Trial Site Pain Points
The article from DIA wraps up with some actionable areas of improvement for relieving clinical trial site burdens, including direct recommendations from the sites.
Single sign-on (SSO) solutions and extending the timeframe before a password expires were at the top of the list for vital—and easy—technology improvements. For site start-up challenges, DIA notes the following improvement opportunities:
“Having well-documented site start-up processes and timelines for each site will also go a long way toward eliminating the burdensome check-ins on status updates and the need to request information that is already clearly captured elsewhere.”
An In-Depth Look at Study Communication and Start-up Challenges
Study start-up is a make-or-break time for the success of a trial. Many sponsors rely on time-consuming, manually created spreadsheets to initiate requests for application access, verify user logins, monitor ongoing access, and manage trials. This hefty administrative burden impedes efficient trial management.
Plus, these outdated methods increase the burdens on sites by contributing to circular email communications to check access status and extensive site user application processes. In a single clinical study, a site user typically engages with seven to 10 systems.
Each of these applications requires setting up two-factor authentication and creating a username, password, and security questions with varying restrictions. Users can spend hours over multiple simultaneous studies just setting up access. Without an SSO or password manager, user fatigue can introduce security risks when staff resort to writing account details on sticky notes or in logbooks.
How to Streamline Clinical Trial Management and Communication
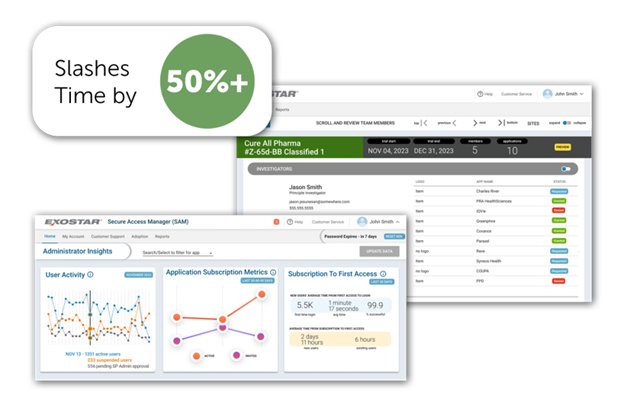
By automating communications and integrating workflows, sponsors can accelerate all stages of trial execution and relieve site burdens. As a recent addition to Exostar’s Secure Access Manager (SAM), Clinical Trial Access Management (CTAM) offers a transformative solution for anyone responsible for managing external user onboarding, including sponsor study managers, clinical research associates, site start-up managers, and local study team representatives.
CTAM provides a real-time administrative insight dashboard to fully manage application access across all studies. With this solution, you can employ user-friendly tools to improve efficiency and better coordinate clinical trial management, including:
- Automated Dashboards: Gain insightful metrics and a comprehensive view of access readiness and utilization trends.
- Integrated Workflows: Manage access requests seamlessly within one integrated workflow involving all stakeholders.
- Real-time Intelligence: Instantly identify and resolve access bottlenecks, gauge site performance, and recognize trends for process improvement.
- Streamlined User Invitations: Accelerate user invitation cycles by searching for and selecting user identities, and integrate learning management system data for streamlined training status tracking.
Solve Top Clinical Trial Challenges with a Proven Solution
Exostar’s SAM is a trusted SSO solution that makes it easy for teams to access all systems associated with clinical trials—internal or external—using one set of login credentials. With the recent addition of CTAM, clinical trial coordination and communication are more transparent than ever with fewer manual actions that produce bottlenecks.
As we saw at DIA 2024 and heard in our meetings at the conference, taking the next step by implementing these solutions delivers efficiency gains for clinical trial sites. Discover today how Secure Access Manager resolves pressing technology challenges by getting in touch with our solution experts.