Blog
Streamlining Clinical Studies: The Urgent Need for a True Single Sign-On (SSO) Solution Across All Sites and Studies

In the rapidly evolving landscape of sponsor-driven clinical studies, the demand for efficient and time-saving solutions has never been more critical. With a shortage of clinical staff, especially since COVID, it becomes imperative to empower the existing workforce to do more in less time.
In this post, we’ll explore the challenges posed by the shortage of clinical staff and how implementing a unique type of Life Sciences SSO – Secure Access Manager (SAM) — can streamline processes, enhance productivity, and ultimately improve the pace of clinical studies.
The Challenge of Clinical Staff Shortages
Clinical studies are at the forefront of medical advancements, and essential to sponsor viability. However, the shortage of clinical staff poses a significant obstacle to the seamless execution of these studies. As the workload on the remaining staff intensifies, finding ways to optimize their workflow becomes paramount. Traditional authentication methods — such as multiple logins and complex password requirements — contribute to unnecessary time delays and can lead to increased stress on already strained resources.
The Case for a Universally Accepted SSO for Life Sciences
Generally, SSO is a technology that allows users to access multiple applications and systems with a single set of credentials. In the context of clinical studies, implementing SSO can alleviate the burden on clinical staff by simplifying the authentication process. Here’s how SSO can make a substantial difference:
- Time Efficiency: Clinical staff often need to access various platforms, databases, and applications throughout the day. With SSO, they can log in once and seamlessly navigate between systems, eliminating the need for repetitive logins and saving valuable time.
- Reduced Cognitive Load: Remembering multiple usernames and passwords for different systems can be mentally taxing. SSO streamlines the authentication process, allowing clinical staff to focus more on their core responsibilities without the distraction of managing numerous credentials.
- Enhanced Security: SSO not only improves efficiency but also enhances security. With a centralized authentication system, organizations can enforce robust security measures, ensuring that sensitive, proprietary data is protected against unauthorized access.
- Adaptability to Mobile Work Environments: Clinical staff are often on the move, requiring access to study data from various locations. SSO facilitates secure and convenient access to critical information, promoting flexibility in work environments.
Why SAM and Its Unique Life Sciences Community Approach is the Ideal SSO Solution for Your Studies
SAM leverages over 650,000 Life Sciences identities across existing sites to unlock greater benefits than standard SSO. A critical challenge to sites is the initial onboarding that takes over 7 minutes per app for each user.
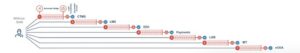
Example: A user onboarding with 7 applications showing 7 minutes per app
Time-saving Impact
Even with a relatively small number of applications per study, each user will accumulate nearly an hour of lost time onboarding per study.
Existing SAM users can drastically reduce this onboarding time and becomes immediately productive; dropping from 49 minutes to 1 minute in total onboarding time. With most sponsors, the number of site users with existing SAM identities will be significant, given our community includes users across 30,000 sites. For brand-new SAM users, they only require a one-time 7-minute onboarding as opposed to 7 minutes for each new app.
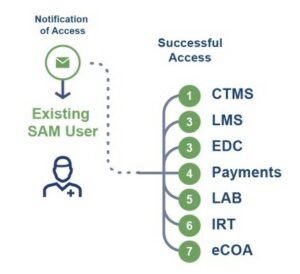
1-minute process to access all study Apps for Existing SAM users
When you consider that the average study has over 11 apps any given user must access, the time adds up quickly. Sponsors can utilize tools to further accelerate this process by delegating its administration to site coordinators, eliminating bottlenecks in assigning access, options for inviting users in bulk, and enabling rapid identification of users that meet their site profile.
Sponsors no longer need to manage these identities and their accompanying baggage and risk. Users no longer get frustrated with a bunch of new passwords and eventual lockouts and lost time.
The financial impacts associated with maintaining multiple passwords grow fast. For example, something as simple as a password reset can cause a company to lose hundreds of thousands of dollars over time. According to Forrester Research, each individual password reset costs $70. Factor in the productivity lost in onboarding for highly paid staff and you have a double-whammy on the bottom line.
Last but not least, productivity loss may mean the difference in meeting a milestone for a trial, which may mean a setback in eventual drug approval. With so much standing in the way of trial performance, it’s crucial to avoid compounding staff shortages with a strategy that doesn’t include universal SSO.
Streamlining Authentication Processes Can Improve Trial Performance
In the face of a shortage of clinical staff, adopting technologies that optimize workflow and enhance productivity is not just a preference but a necessity. A Single Sign-On (SSO) solution such as SAM that leverages a large existing pool of Life Sciences users can play a key role in addressing the challenges posed by the current study landscape. By streamlining authentication processes and reducing the cognitive load on staff, SSO empowers the existing workforce to contribute more efficiently to the progress of clinical studies. As trials continue to evolve, embracing technologies like SSO becomes a strategic imperative, ensuring that clinical studies can proceed at an accelerated pace without compromising data security or the well-being of the dedicated professionals at the heart of these critical initiatives.
More Resources
Contact our team of experts to learn how Exostar’s SAM can help revolutionize your clinical trials as the only SSO solution that combines subject matter expertise, vertical market experience, and broad acceptance in the Life Sciences industry.
It will be our pleasure to conduct an analysis and show how our unique SAM Life Sciences Community can help you.
About the Author
Martin O’Malley is the Director of Life Sciences at Exostar and has spent his 20-year career optimizing clinical workflow with the application of digital solutions. Martin is a member of several nationally recognized health data organizations and attended Tulane School of Law.